COVID-19 has wreaked havoc across the globe destroying livelihoods, businesses, economies but nothing is more precious than our health.
At the time of writing, over a million people have died with over 40 million confirmed cases worldwide. In the UK alone nearly 44,000 people have sadly lost their lives with nearly 800,000 confirmed cases since the outbreak began earlier this year.
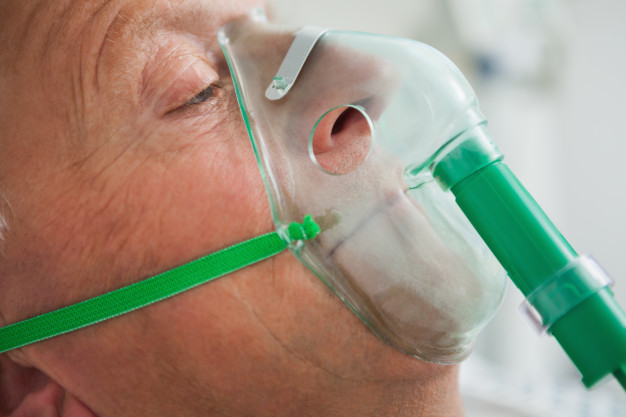
COVID-19 attacks the tissues and airways deep inside the lungs. The disease can progress to viral pneumonia which is a real worry for those already suffering from an immune deficiency or a respiratory ailment such as COPD or Asthma. It can cause inflammation of the lungs effecting the alveoli and the transfer of oxygen to red blood cells.
The world has learnt a lot since the first wave of the virus with new treatments and drugs being trialed and tested with the prospect of a vaccine on the horizon. However, Oxygen therapy has been and continues to be a major treatment intervention for patients with severe COVID-19 according to a recent publication by the World Health Organisation.
As pneumonia hinders the transfer of Oxygen into our bloodstream, for those who are particularly vulnerable, using an oxygen concentrator can support lung function by keeping red blood cells saturated as best it can until the infection passes.
Visit our dedicated Oxygen Concentrators website here to learn more
References: https://www.bbc.co.uk/news/health-51214864 ~ https://www.telegraph.co.uk/global-health/science-and-disease/coronavirus-symptoms-covid-19-help-advice/