The store will not work correctly in the case when cookies are disabled.
JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser.
NHS Approved Supplier We accept NHS purchase orders
Established Since 1997 Your trusted medical equipment partner
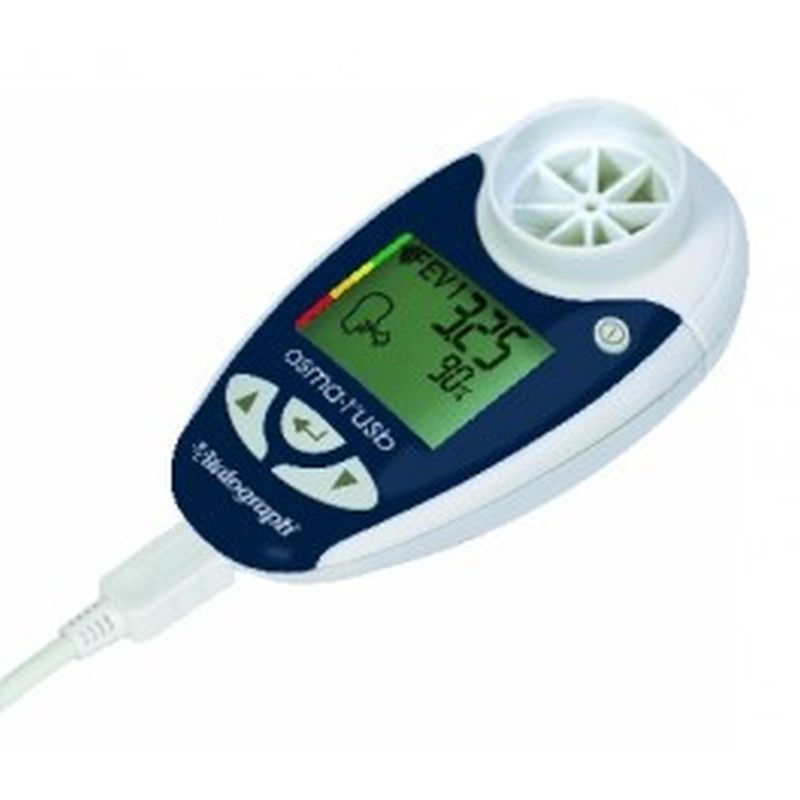
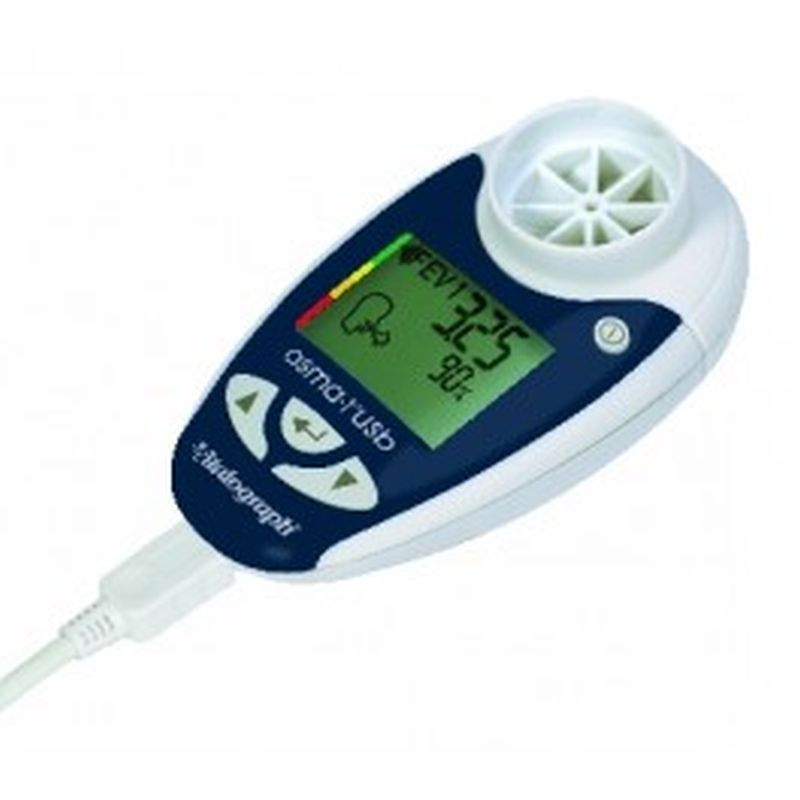
Vitalograph Respiratory Monitor asma-1 USB The asma-1™ USB combines all the features of the asma-1 with the ability to link to your PC. Session data and device ID may be transmitted to Vitalograph Reports, Spirotrac or an API developers' kit is available for providers of e-Diary, home hub and telemedicine solutions.
Features:
Indicates blow quality, number of blows and diurnal variation
Device ID automatically transmitted with the data
Ability to add comments to the report
Optionally separate graphs of am and pm sessions
Information presented in tabular and graphical form
Can automatically name the PDF file
Subject ID, name, age, height, gender and optional weight field
Time/date stamp for each session
Best PEF and FEV1 fields automatically populated
BMI calculated (from height and weight)
Personal best FEV1, PEF and zones as set in asma-1
Trend graphs of FEV1 and PEF
This is usually a stock item - Please allow 1 to 3 working days delivery
In most cases, items in stock that are ordered before 12 noon are delivered on a next working day service.
If you need this item urgently please use the live chat or call us to enquire about our premium rate delivery service including same-day, timed and weekend deliveries. For more information about delivery, please visit our delivery information page here
Brand - Vitalograph
Detailed Technical Information -
Product
Vitalograph asma-1
Model Number
4000
Part Number
40000, 40400, 40300, 40050
Description
Vitalograph asma-1, Vitalograph asma-1 USB, Vitalograph asma-1 Bluetooth, Vitalograph asma-1 Serial
Size
113 x 63 x 48mm
Weight
55g net
Parameters Displayed (Adult version)
PEF & FEV1
Parameters Displayed (Child version)
PEF, FEV0.5, FEV0.75 and FEV1
Operating Temperature Range
17 - 37°C
Measuring Principle
Stator/rotor mass flow meter
Scale Intervals
PEF 1L/min
Accuracy
FEV1 better than +/-3% or +/-100ml (whichever is greater)
Repeatability
Better than 5% or 10L/min, whichever is greater
Cleaning
Alcohol wipe, especially the mouthpiece, weekly
Flow Range
0 - 999 L/min
Disposal Methods
Recycle
Performance standard
ISO 26782:2009; ISO 23747:2007; ATS/ERS 2005
Safety Standards
IEC 60601-1:2005
Designed and manufactured under
CE 0086
Vitalograph Respiratory Monitor asma-1 USB SKU: VT-40400
Price:
£188.40 £157.00
Description
Specification ss https://www.portableoxygen.co.uk/intermedicaladmin/cms/wysiwyg/directive/___directive/e3ttZWRpYSB1cmw9Ijx0YWJsZT4NCjx0Ym9keT4NCjx0cj4NCjx0ZD5Qcm9kdWN0PC90ZD4NCjx0ZD5WaXRhbG9ncmFwaCBhc21hLTE8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5Nb2RlbCBOdW1iZXI8L3RkPg0KPHRkPjQwMDA8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5QYXJ0IE51bWJlcjwvdGQ-DQo8dGQ-NDAwMDAsIDQwNDAwLCA0MDMwMCwgNDAwNTA8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5EZXNjcmlwdGlvbjwvdGQ-DQo8dGQ-Vml0YWxvZ3JhcGggYXNtYS0xLCBWaXRhbG9ncmFwaCBhc21hLTEgVVNCLCBWaXRhbG9ncmFwaCBhc21hLTEgQmx1ZXRvb3RoLCBWaXRhbG9ncmFwaCBhc21hLTEgU2VyaWFsPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-U2l6ZTwvdGQ-DQo8dGQ-MTEzIHggNjMgeCA0OG1tPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-V2VpZ2h0PC90ZD4NCjx0ZD41NWcgbmV0PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-UGFyYW1ldGVycyBEaXNwbGF5ZWQgKEFkdWx0IHZlcnNpb24pPC90ZD4NCjx0ZD5QRUYgJiBGRVYxPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-UGFyYW1ldGVycyBEaXNwbGF5ZWQgKENoaWxkIHZlcnNpb24pPC90ZD4NCjx0ZD5QRUYsIEZFVjAuNSwgRkVWMC43NSBhbmQgRkVWMTwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPk9wZXJhdGluZyBUZW1wZXJhdHVyZSBSYW5nZTwvdGQ-DQo8dGQ-MTcgLSAzNyZkZWc7QzwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPk1lYXN1cmluZyBQcmluY2lwbGU8L3RkPg0KPHRkPlN0YXRvci9yb3RvciBtYXNzIGZsb3cgbWV0ZXI8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5TY2FsZSBJbnRlcnZhbHM8L3RkPg0KPHRkPlBFRiAxTC9taW4gPGJyIC8-RkVWMSAxTDwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPkFjY3VyYWN5PC90ZD4NCjx0ZD5GRVYxIGJldHRlciB0aGFuICsvLTMlIG9yICsvLTEwMG1sICh3aGljaGV2ZXIgaXMgZ3JlYXRlcikgPGJyIC8-UEVGIGJldHRlciB0aGFuICsvLTUlIG9yICsvLTI1TC9taW4gKHdoaWNoZXZlciBpcyBncmVhdGVyKTwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPlJlcGVhdGFiaWxpdHk8L3RkPg0KPHRkPkJldHRlciB0aGFuIDUlIG9yIDEwTC9taW4sIHdoaWNoZXZlciBpcyBncmVhdGVyPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-Q2xlYW5pbmc8L3RkPg0KPHRkPkFsY29ob2wgd2lwZSwgZXNwZWNpYWxseSB0aGUgbW91dGhwaWVjZSwgd2Vla2x5PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-RmxvdyBSYW5nZTwvdGQ-DQo8dGQ-MCAtIDk5OSBML21pbjwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPkRpc3Bvc2FsIE1ldGhvZHM8L3RkPg0KPHRkPlJlY3ljbGU8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5QZXJmb3JtYW5jZSBzdGFuZGFyZDwvdGQ-DQo8dGQ-SVNPIDI2NzgyOjIwMDk7IElTTyAyMzc0NzoyMDA3OyBBVFMvRVJTIDIwMDU8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5TYWZldHkgU3RhbmRhcmRzPC90ZD4NCjx0ZD5JRUMgNjA2MDEtMToyMDA1PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-RGVzaWduZWQgYW5kIG1hbnVmYWN0dXJlZCB1bmRlcjwvdGQ-DQo8dGQ-Q0UgMDA4NiA8YnIgLz5JU08gMTM0ODU6MjAwMyA8YnIgLz5GREEgMjFDRlI4MjA8L3RkPg0KPC90cj4NCjwvdGJvZHk-DQo8L3RhYmxlPiJ9fQ,,/key/8370da046b704174b2aae31670af49f257ce5dbc05c193cac0ebfdccf4786fce/
Images VT-40400.jpg
VT-40400.jpg
Medical Equipment Supplies Experts With dedicated support and service
Secure Payment Payments secured by SagePay, PayPal or Amazon Pay
Buy now, Pay in 30 days Credit accounts available to NHS organisations and limited companies. Register here
Disposables and Newsletter Enter your details to receive the latest updates
Cookies and Privacy
This website use cookies to ensure you get the best user experience on our website.
Cookie Settings





 VT-40400.jpg
VT-40400.jpg 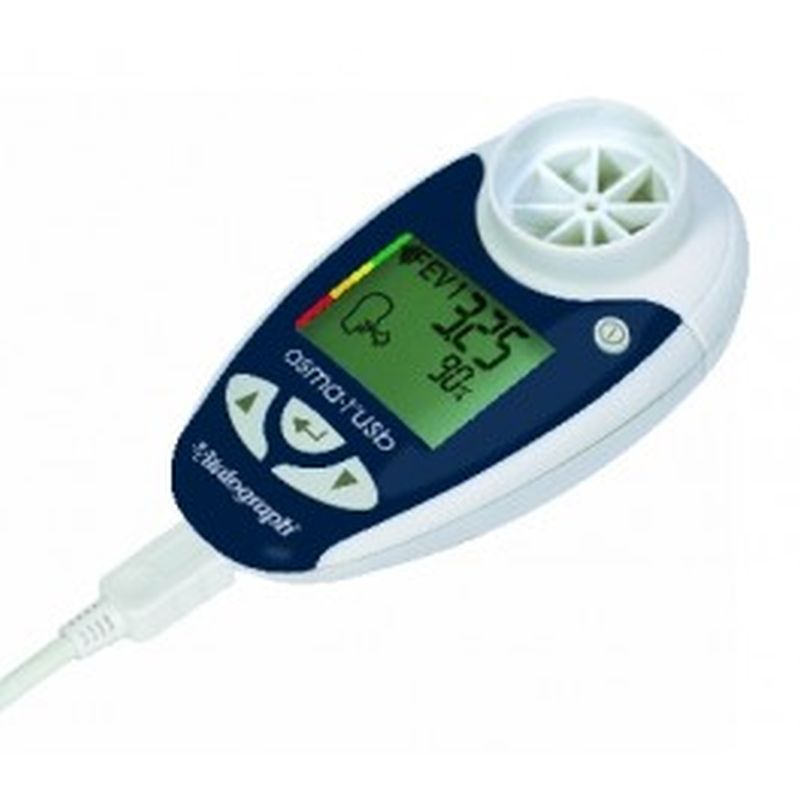 VT-40400.jpg
VT-40400.jpg 





