The store will not work correctly in the case when cookies are disabled.
JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser.
NHS Approved Supplier We accept NHS purchase orders
Established Since 1997 Your trusted medical equipment partner
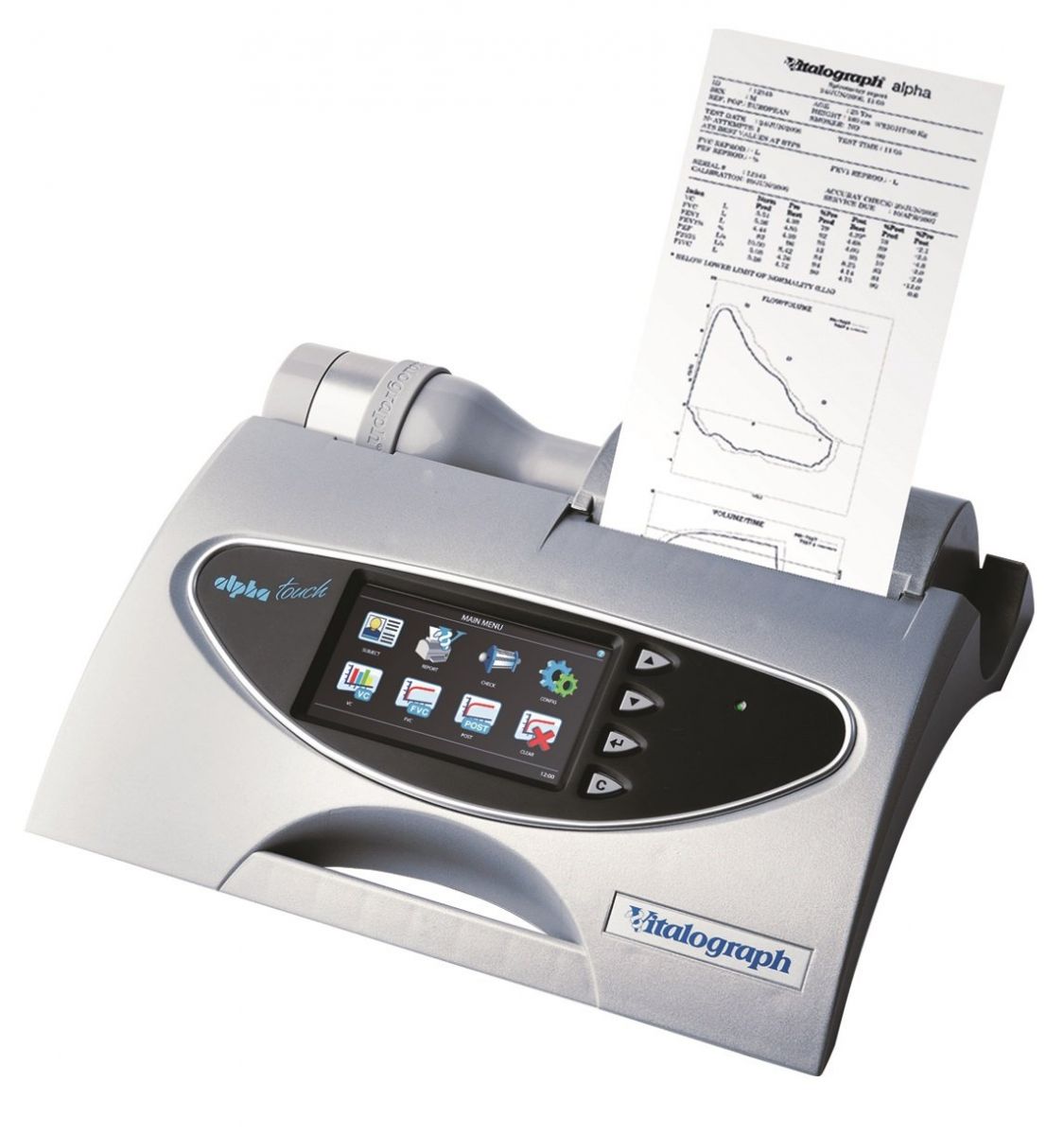
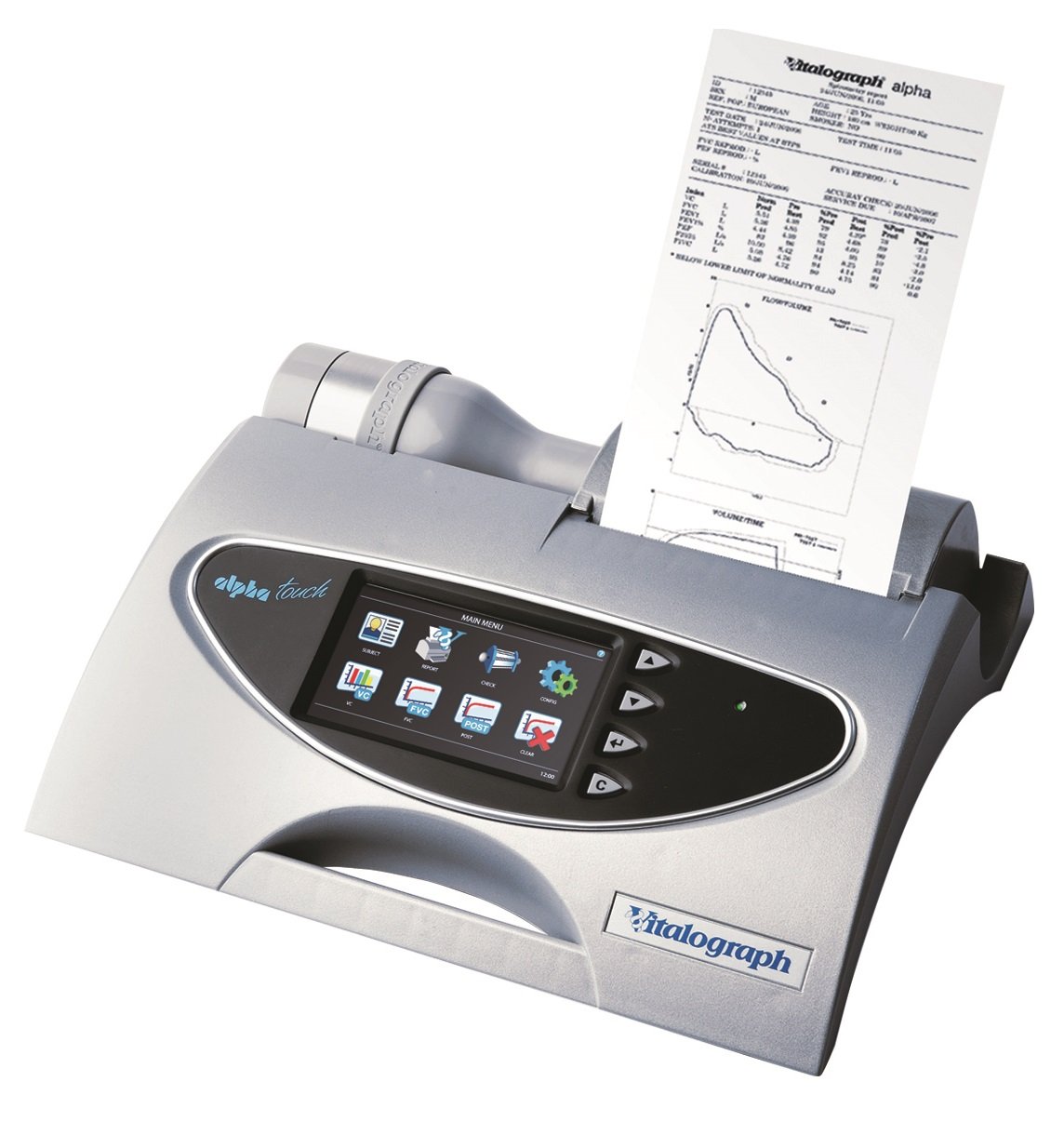
Vitalograph Alpha Touch Spirometer with Vitalograph Reports software
In2itive Spirometer
The In2itive is a lightweight handheld, fully functional spirometer, providing portability in addition to the reporting capability of a networkable PC spirometer.
Ideal for use on multiple subjects within general healthcare and occupational health environments. Perfectly suited for both adult and pediatric testing with data storage for over 10,000 subjects. A color touch screen display and an intuitive icon driven menu, makes the In2itive quick and simple to operate. Simple, validated hygiene system using Bacterial Viral Filters means that it is easy and inexpensive to keep the device clean.
The In2itive Spirometer comes with:
Cradle
Remote Flowhead
USB cable
Charger plug
Stylus
Vitalograph Reports Software
Carry Pouch
Spirotrac Software (Optional)
Capabilities
VC, FVC, and bronchodilator responsiveness testing. Over 50 available parameters, GLI predicted equations and Z-scores.
Low running costs. No need for costly disposable sensors, turbines, or flow tubes.
Save time & money on cleaning by using Vitalograph Bacterial Viral Filters (BVFTM).
Real-time curves and incentive animations encourage optimal performance.
Remote flowhead enables the subject to view incentives while performing the test.
Robust, linear and reliable flow measurements even at very low flow rates.
Cradle for battery charging, connection to a PC and automatic data exchange.
Be sure of accurate test results through quick and easy calibration verification routines as recommended by international spirometry guidelines (ATS/ERS and ARTP).
Electrical outlet or rechargeable battery powered for total flexibility and portability.
This is usually a stock item - Please allow 1 to 3 working days delivery
In most cases, items in stock that are ordered before 12 noon are delivered on a next working day service.
If you need this item urgently please use the live chat or call us to enquire about our premium rate delivery service including same-day, timed and weekend deliveries. For more information about delivery, please visit our delivery information page here
Brand - Vitalograph
Detailed Technical Information -
Product
Vitalograph ALPHA Touch
Model Number
6000
Dimensions
300mm x 250mm x 75mm
Weight
2kg net
Gross Weight and Size (Packed)
470mm x 112mm x 377mm 4kg
Subjects
Automatic population of database from Spirotrac. Name, ID; Age, Height, Gender; Smoking Status, Body Mass Index and more depending on model. Storage of up to 10,000 subjects
Selectable Parameters (depending on model)
VC; IVC; IC; VT (TV); TLC; RV; IRV; ERV; FRC; FVC; FIVC; FEV1; FEV3; FEV6; FVC; FEV1/VC; FEV1/FVC; FEV3/VC; FEV3/FVC; FEV1/FEV6; FEF75; FEF50; FEF25; FEF25-75; FEF25-75/FVC; FIV1; PIF, FIV1/FIVC, FIF25, FIF50, FIF75, FEF50/FIF50, FET, MVVind, FEV1 Ratio; FEV0.5; PEF L/min; PEF L/s; FEF 0.2-1.2; FEF 75-85%; FEF25%; FEF50%; FEF75%; FMFT; FIF25%; FIF50%; FIF75%; PIF; FEV0.75; Lung Age and more
Environmental Data
Selectable: Temperature (built in sensor), Barometric pressure, altitude and humidity
Test Types
Single breath tests, flow/volume loops, multi-breath testing, tidal breathing and combined VC/FVC type test methods supported
Predicted Values Selectable (depending on model)
ERS/ECCS; GLI, NHANES, Polgar, Pereira, Berglund, Forsche, Gutierrez, Hedenström, Taiwan; Knudson, Crapo; Hsu; KNLW; Viljanen; SEPAR; Gulsvik; SBPT; Tamura; Ip; Quanjer; Wang; Dockery and more
Suggested Interpretation
User selectable
Storage Humidity
10% to 95%
Storage Temperature
0°C to 50°C
Recommended Operating Temperature Range
17 - 37°C; Design Limits: 10-40°C
Data Storage
Stores up to 10,000 subjects
PowerSAFE
Input 100 - 240V AC 50-60Hz, output 12V DC
Battery Pack
Rechargeable NiMh, 7.2V 1, 800mAH
Flow Detection Principle
Fleisch type pneumotachograph
Volume Detection
Flow integration sampling @ 100Hz
Resolution
10 mL volume; 0.01 L/s flow
Accuracy when in Operating Range
Flow +/- from 10% to 5%
Volume Accuracy
Better than +/-3
Maximum Displayed Volume
10L
Linearity
+/-3%
Maximum Test Duration
90 seconds
Flow Impedance
0.1kpa L/s @ 14L/s
Printer
Thermal
Performance Standards
ISO 26782:2009
Safety Standards
IEC 60601-1:2005
Medical Devices Directive
93/42/EEC1993 as amended
Designed & Manufactured Under
ISO 13485:2003 and 2016
Communications
USB and CF card
Connectivity
USB 2 B
Vitalograph Alpha Touch Spirometer with Vitalograph Reports software SKU: VT-65502
Price:
£1,620.00 £1,350.00
Description
Specification ss https://www.portableoxygen.co.uk/intermedicaladmin/cms/wysiwyg/directive/___directive/e3ttZWRpYSB1cmw9Ijx0YWJsZT4NCjx0Ym9keT4NCjx0cj4NCjx0ZD5Qcm9kdWN0PC90ZD4NCjx0ZD5WaXRhbG9ncmFwaCBBTFBIQSBUb3VjaDwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPk1vZGVsIE51bWJlcjwvdGQ-DQo8dGQ-NjAwMDwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPkRpbWVuc2lvbnM8L3RkPg0KPHRkPjMwMG1tIHggMjUwbW0geCA3NW1tPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-V2VpZ2h0PC90ZD4NCjx0ZD4ya2cgbmV0PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-R3Jvc3MgV2VpZ2h0IGFuZCBTaXplIChQYWNrZWQpPC90ZD4NCjx0ZD40NzBtbSB4IDExMm1tIHggMzc3bW0gNGtnPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-U3ViamVjdHM8L3RkPg0KPHRkPkF1dG9tYXRpYyBwb3B1bGF0aW9uIG9mIGRhdGFiYXNlIGZyb20gU3Bpcm90cmFjLiBOYW1lLCBJRDsgQWdlLCBIZWlnaHQsIEdlbmRlcjsgU21va2luZyBTdGF0dXMsIEJvZHkgTWFzcyBJbmRleCBhbmQgbW9yZSBkZXBlbmRpbmcgb24gbW9kZWwuIFN0b3JhZ2Ugb2YgdXAgdG8gMTAsMDAwIHN1YmplY3RzPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-U2VsZWN0YWJsZSBQYXJhbWV0ZXJzIChkZXBlbmRpbmcgb24gbW9kZWwpPC90ZD4NCjx0ZD5WQzsgSVZDOyBJQzsgVlQgKFRWKTsgVExDOyBSVjsgSVJWOyBFUlY7IEZSQzsgRlZDOyBGSVZDOyBGRVYxOyBGRVYzOyBGRVY2OyBGVkM7IEZFVjEvVkM7IEZFVjEvRlZDOyBGRVYzL1ZDOyBGRVYzL0ZWQzsgRkVWMS9GRVY2OyBGRUY3NTsgRkVGNTA7IEZFRjI1OyBGRUYyNS03NTsgRkVGMjUtNzUvRlZDOyBGSVYxOyBQSUYsIEZJVjEvRklWQywgRklGMjUsIEZJRjUwLCBGSUY3NSwgRkVGNTAvRklGNTAsIEZFVCwgTVZWaW5kLCBGRVYxIFJhdGlvOyBGRVYwLjU7IFBFRiBML21pbjsgUEVGIEwvczsgRkVGIDAuMi0xLjI7IEZFRiA3NS04NSU7IEZFRjI1JTsgRkVGNTAlOyBGRUY3NSU7IEZNRlQ7IEZJRjI1JTsgRklGNTAlOyBGSUY3NSU7IFBJRjsgRkVWMC43NTsgTHVuZyBBZ2UgYW5kIG1vcmU8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5FbnZpcm9ubWVudGFsIERhdGE8L3RkPg0KPHRkPlNlbGVjdGFibGU6IFRlbXBlcmF0dXJlIChidWlsdCBpbiBzZW5zb3IpLCBCYXJvbWV0cmljIHByZXNzdXJlLCBhbHRpdHVkZSBhbmQgaHVtaWRpdHk8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5UZXN0IFR5cGVzPC90ZD4NCjx0ZD5TaW5nbGUgYnJlYXRoIHRlc3RzLCBmbG93L3ZvbHVtZSBsb29wcywgbXVsdGktYnJlYXRoIHRlc3RpbmcsIHRpZGFsIGJyZWF0aGluZyBhbmQgY29tYmluZWQgVkMvRlZDIHR5cGUgdGVzdCBtZXRob2RzIHN1cHBvcnRlZDwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPlByZWRpY3RlZCBWYWx1ZXMgU2VsZWN0YWJsZSAoZGVwZW5kaW5nIG9uIG1vZGVsKTwvdGQ-DQo8dGQ-RVJTL0VDQ1M7IEdMSSwgTkhBTkVTLCBQb2xnYXIsIFBlcmVpcmEsIEJlcmdsdW5kLCBGb3JzY2hlLCBHdXRpZXJyZXosIEhlZGVuc3RyJm91bWw7bSwgVGFpd2FuOyBLbnVkc29uLCBDcmFwbzsgSHN1OyBLTkxXOyBWaWxqYW5lbjsgU0VQQVI7IEd1bHN2aWs7IFNCUFQ7IFRhbXVyYTsgSXA7IFF1YW5qZXI7IFdhbmc7IERvY2tlcnkgYW5kIG1vcmU8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5TdWdnZXN0ZWQgSW50ZXJwcmV0YXRpb248L3RkPg0KPHRkPlVzZXIgc2VsZWN0YWJsZTwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPlN0b3JhZ2UgSHVtaWRpdHk8L3RkPg0KPHRkPjEwJSB0byA5NSU8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5TdG9yYWdlIFRlbXBlcmF0dXJlPC90ZD4NCjx0ZD4wJmRlZztDIHRvIDUwJmRlZztDPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-UmVjb21tZW5kZWQgT3BlcmF0aW5nIFRlbXBlcmF0dXJlIFJhbmdlPC90ZD4NCjx0ZD4xNyAtIDM3JmRlZztDOyBEZXNpZ24gTGltaXRzOiAxMC00MCZkZWc7QzwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPkRhdGEgU3RvcmFnZTwvdGQ-DQo8dGQ-U3RvcmVzIHVwIHRvIDEwLDAwMCBzdWJqZWN0czwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPlBvd2VyU0FGRTwvdGQ-DQo8dGQ-SW5wdXQgMTAwIC0gMjQwViBBQyA1MC02MEh6LCBvdXRwdXQgMTJWIERDPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-QmF0dGVyeSBQYWNrPC90ZD4NCjx0ZD5SZWNoYXJnZWFibGUgTmlNaCwgNy4yViAxLCA4MDBtQUg8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5GbG93IERldGVjdGlvbiBQcmluY2lwbGU8L3RkPg0KPHRkPkZsZWlzY2ggdHlwZSBwbmV1bW90YWNob2dyYXBoPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-Vm9sdW1lIERldGVjdGlvbjwvdGQ-DQo8dGQ-RmxvdyBpbnRlZ3JhdGlvbiBzYW1wbGluZyBAIDEwMEh6PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-UmVzb2x1dGlvbjwvdGQ-DQo8dGQ-MTAgbUwgdm9sdW1lOyAwLjAxIEwvcyBmbG93PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-QWNjdXJhY3kgd2hlbiBpbiBPcGVyYXRpbmcgUmFuZ2U8L3RkPg0KPHRkPkZsb3cgKy8tIGZyb20gMTAlIHRvIDUlIDxiciAvPk1heCBmbG93IHJhdGUgKy8tMTZML3MgPGJyIC8-TWluIGZsb3cgcmF0ZSArLy0wLjAyTC9zPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-Vm9sdW1lIEFjY3VyYWN5PC90ZD4NCjx0ZD5CZXR0ZXIgdGhhbiArLy0zPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-TWF4aW11bSBEaXNwbGF5ZWQgVm9sdW1lPC90ZD4NCjx0ZD4xMEw8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5MaW5lYXJpdHk8L3RkPg0KPHRkPisvLTMlPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-TWF4aW11bSBUZXN0IER1cmF0aW9uPC90ZD4NCjx0ZD45MCBzZWNvbmRzPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-RmxvdyBJbXBlZGFuY2U8L3RkPg0KPHRkPjAuMWtwYSBML3MgQCAxNEwvcyA8YnIgLz5Db21wbGllcyB3aXRoIEFUUy9FUlMgMjAwNTwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPlByaW50ZXI8L3RkPg0KPHRkPlRoZXJtYWw8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5QZXJmb3JtYW5jZSBTdGFuZGFyZHM8L3RkPg0KPHRkPklTTyAyNjc4MjoyMDA5PGJyIC8-SVNPIDIzNzQ3OjIwMDcgPGJyIC8-QVRTL0VSUyAyMDA1PC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-U2FmZXR5IFN0YW5kYXJkczwvdGQ-DQo8dGQ-SUVDIDYwNjAxLTE6MjAwNTwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPk1lZGljYWwgRGV2aWNlcyBEaXJlY3RpdmU8L3RkPg0KPHRkPjkzLzQyL0VFQzE5OTMgYXMgYW1lbmRlZDwvdGQ-DQo8L3RyPg0KPHRyPg0KPHRkPkRlc2lnbmVkICYgTWFudWZhY3R1cmVkIFVuZGVyPC90ZD4NCjx0ZD5JU08gMTM0ODU6MjAwMyBhbmQgMjAxNjxiciAvPkZEQSAyMSBDRlIgODIwIDxiciAvPkNNRFI8L3RkPg0KPC90cj4NCjx0cj4NCjx0ZD5Db21tdW5pY2F0aW9uczwvdGQ-DQo8dGQ-VVNCIGFuZCBDRiBjYXJkPC90ZD4NCjwvdHI-DQo8dHI-DQo8dGQ-Q29ubmVjdGl2aXR5PC90ZD4NCjx0ZD5VU0IgMiBCPC90ZD4NCjwvdHI-DQo8L3Rib2R5Pg0KPC90YWJsZT4ifX0,/key/ea7a7b81512c44d08e6a4f2852de80499c118515330b1db8e449e6361c0478b5/
Images VT-65502.jpg
VT-65502.jpg
Medical Equipment Supplies Experts With dedicated support and service
Secure Payment Payments secured by SagePay, PayPal or Amazon Pay
Buy now, Pay in 30 days Credit accounts available to NHS organisations and limited companies. Register here
Disposables and Newsletter Enter your details to receive the latest updates
Cookies and Privacy
This website use cookies to ensure you get the best user experience on our website.
Cookie Settings





 VT-65502.jpg
VT-65502.jpg 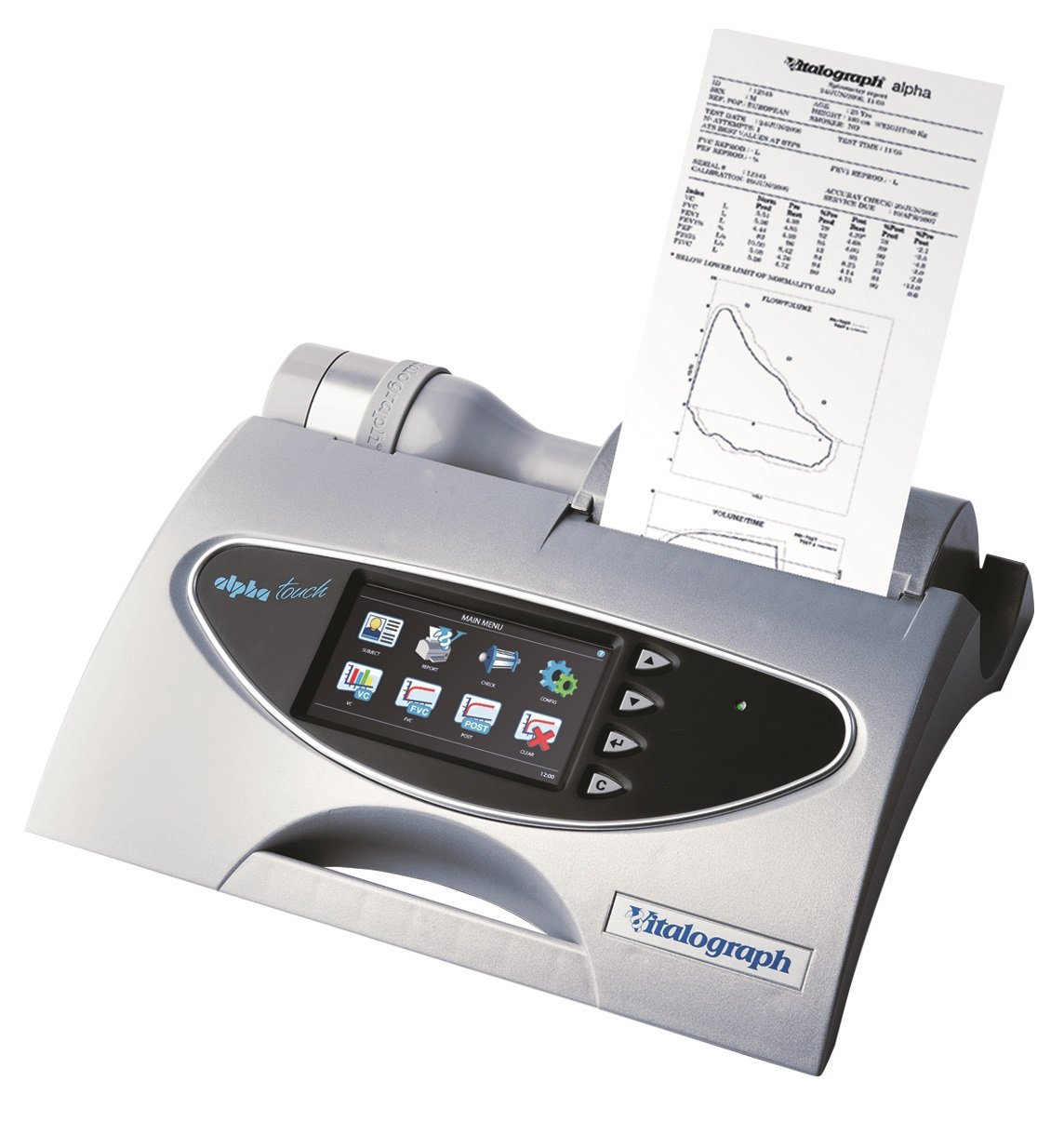 VT-65502.jpg
VT-65502.jpg 





