We accept NHS purchase orders

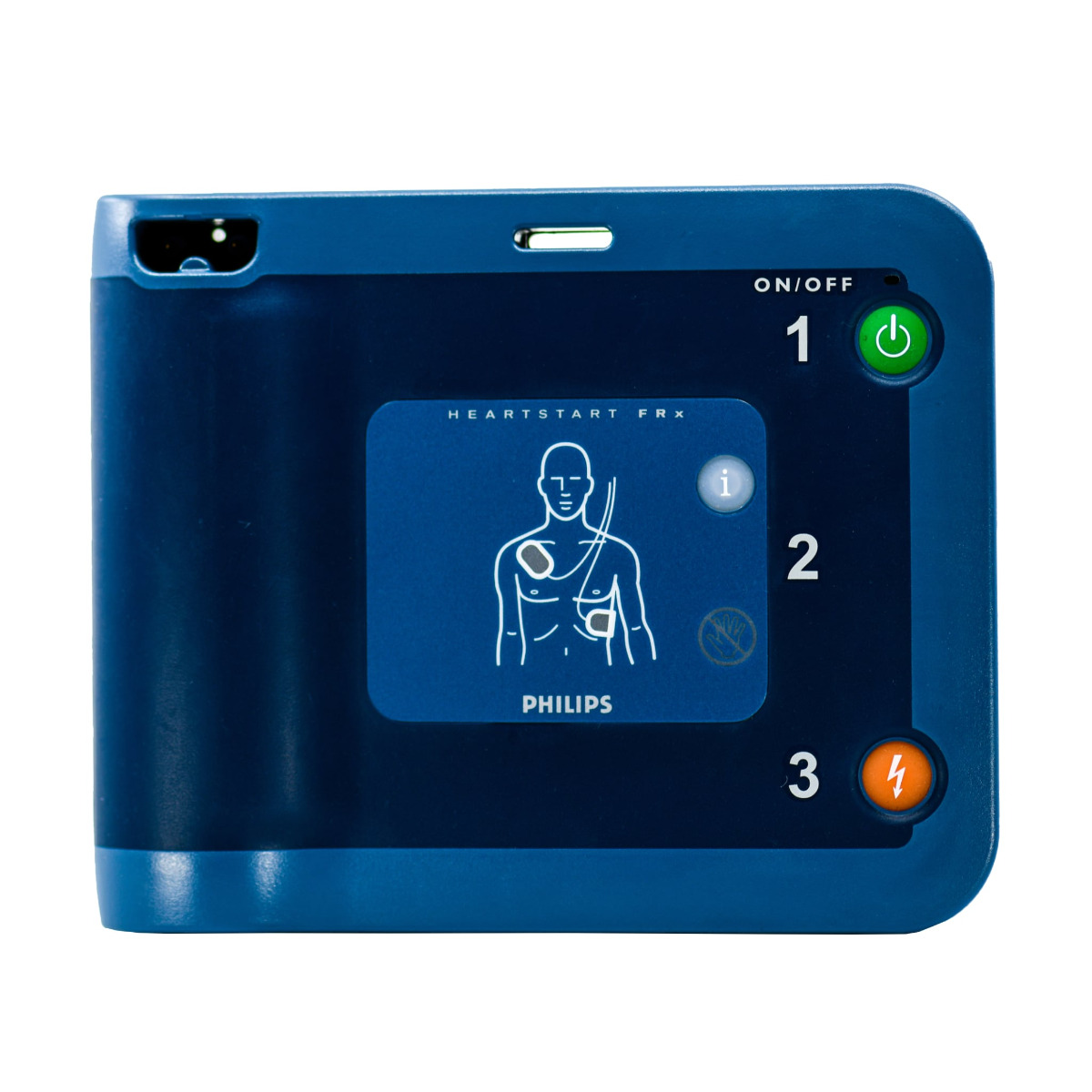
Philips HeartStart FRx Defibrillator with Pads
If you are looking to place an order of significant quantity for this item, we may be able to help you with a bespoke quote.
Please complete all the details required below.
The Philips HeartStart FRx AED
- Is lightweight, rugged and reliable
- Includes features to help guide the treatment of SCA with easy setup, real-time metronomeand clear, step-by-step voice commands paced to your actions
- Provides CPR instructions for infants and children under 25 kg (55 lb) or 0-8 years old, and adults and children over 25 kg (55 lb) or greater than 8 years old
- Has an optional Infant/Child Key; simply insert it and the defibrillator adjusts instruction and therapy, eliminating the need for additional infant/child pads
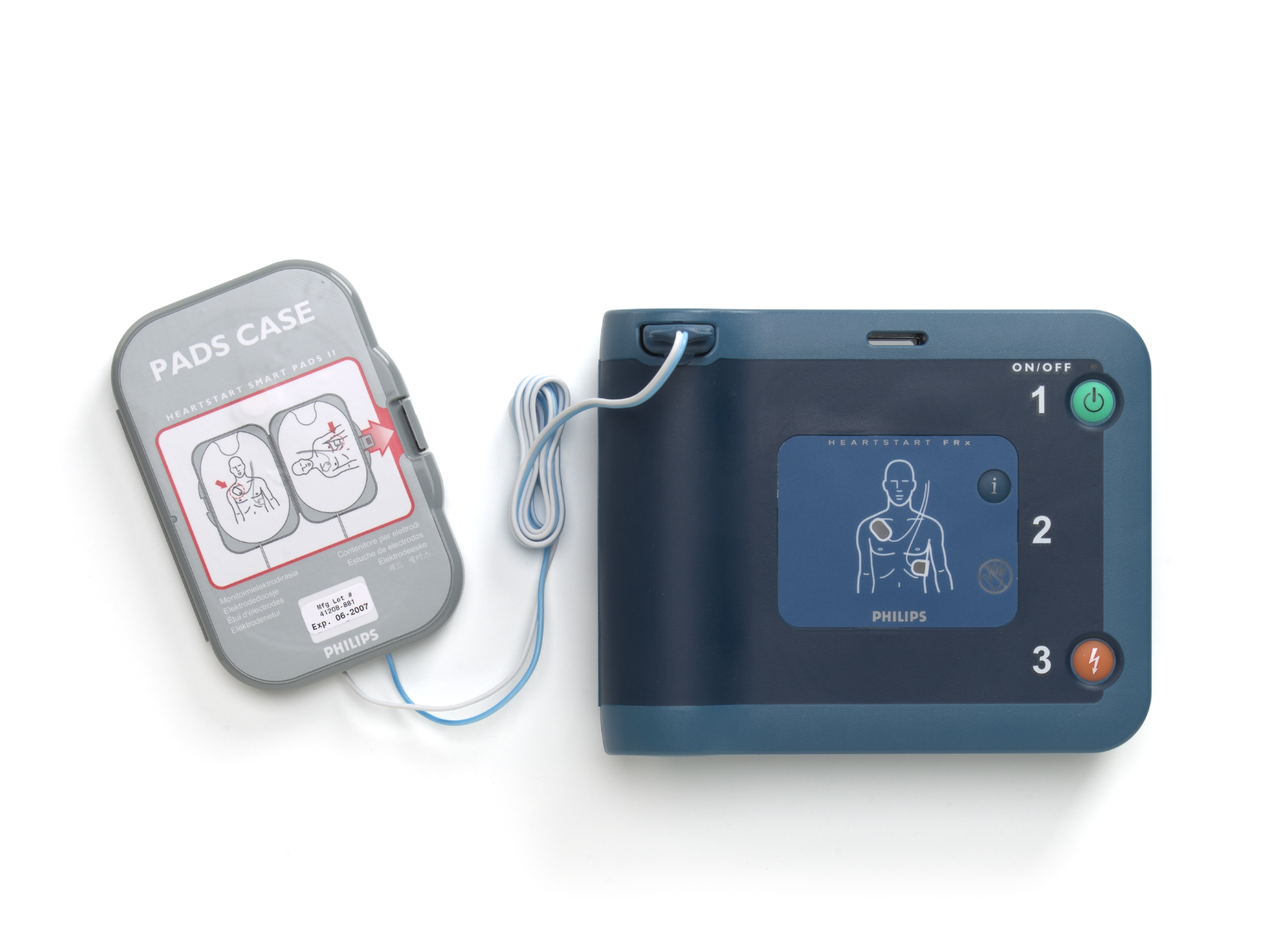
- Includes pre-connected SMART Pads II that can be used for both adults and children; SMART Analysis automatically assesses heart rhythm and only delivers a shock if the victim’s rhythm is determined to be treatable by the Philips advanced algorithm, even if the Shock button is pressed
- Has patented Quick Shock feature that allows the FRx to typically deliver a shock within eight seconds after CPR2
- Performs a series of automatic self-tests daily, weekly and monthly to check pad readiness and verify functionality and calibration of circuits and systems
The FRx makes training easy: Simply insert the Training Pads II (available separately) to temporarily
disable the FRx’s energy delivery capability and switch into training mode. Eight realistic SCA scenarios are designed to keep you and your team confident and prepared when the moment arises.
Stock items are usually dispatched and delivered within 1 to 3 working days.
In most cases, items in stock that are ordered before 12 noon are delivered on a next working day service.
For accurate delivery information, please call 0845 862 9500 or press the live chat button to speak to one of our online sales team.
If you need this item urgently please contact us using the live chat or call us on 0845 862 9500 to enquire about our premium rate delivery service including same-day, timed and weekend deliveries.
For more information about delivery, please visit our delivery information page here
Defibrillator
HeartStart FRx AED specifications:
Model number: 861304
Includes defibrillator, battery, SMART Pads II (1 set), Setup and Maintenance Guides, Owner’s Manual, Quick Reference Guide, date sticker
Waveform: Biphasic truncated exponential; waveform parameters are automatically adjusted as a
function of patient defibrillation impedance
Therapy: Adult defibrillation: nominal peak current 32 A (150 J nominal into a 50 ohm load) Pediatric defibrillation with optional FRx Infant/Child Key installed: nominal peak current 19 A (50 J nominal into 50 ohm load)
Protocol: Device follows preconfigured settings; defibrillation and CPR protocol can be customized using HeartStart Event Review Pro software
User interface
Instructions: Detailed voice prompts and visual icons guide responder through use of the defibrillator
CPR guidance: Verbal instructions for adult and infant/child CPR provide instructions and audio cues for the appropriate number, rate and depth of chest compressions, as well as for each breath
Controls: Green On/Off button, blue-lit i-button, orange Shock button, optional Infant/Child Key Indicators Ready light, blue-lit i-button, caution light, illuminated pads, icons; Shock button lights up when shock is advised
Physical
Size: 18 x 6 x 22 cm (7.1 x 2.4 x 8.8 in) H x D x W
Weight: Approximately 1.6 kg (3.5 lb) with battery and pads installed
Environmental/physical requirements
Sealing: Waterjet-proof IPX5 per IEC60529
Dust-protected IP5X per IEC60529
Temperature: Operating/Standby: 0° – 50° C (32° – 122° F)
Transient operating (for 20 minutes or less, after rapid transition from 20° C [68° F]): -20 to 50° C (-4° to 122° F); under non-condensing humidity conditions
Altitude: 0 to 4,572 m (0 to 15,000 ft)
Aircraft: Meets RTCA/DO-160 Section 21 (Category M – Radiated Emissions) and Section 20
(Category M – Conducted Immunity, and Category D – Radiated Immunity)
Crush: 500 kg (1100 lb)
Drop: Withstands 1.22 m (4 ft) drop on any edge, corner or face of the device onto masonry surface
Vibration: Operating: meets MILSTD 810G Fig. 5146E-1, random
Standby: meets MILSTD 810G Fig. 5146E-2, swept sine (helicopter)
EMI: (radiated/ immunity) Meets CISPR 11 Group 1 Class B and IEC 61000-4-3
Data recording and transmission
Infrared: Wireless transmission of event data to a PC using the IrDA protocol
HeartStart Event Review Pro software: Data management software (optional) for download and review of data retrieved through defibrillator’s infrared data port
Data stored: First 15 minutes of ECG and the entire incident’s events and analysis decisions
Patient analysis system
Patient analysis: Evaluates patient ECG to determine if a rhythm is shockable. Rhythms considered shockable are ventricular fibrillation (VF) and certain ventricular tachycardias (VT) associated with lack of circulation. For safety reasons, some VT rhythms associated with circulation will not be interpreted as shockable, and some very low-amplitude or low-frequency rhythms will not be interpreted as shockable VF.
Sensitivity/specificity: Meets AAMI DF80 guidelines and AHA recommendations for adult defibrillation
Shock advised: Able to deliver a shock as soon as the device indicates a shock is advised
Quick Shock: Able to deliver a shock after the last chest compression of a CPR interval, typically in 8 seconds
Shock-to-shock cycle time: Typically less than 20 seconds between shocks in a series
Artifact detection: Allows ECG analysis even in the presence of most pacemaker artifact and electrical noise sources; other artifacts are detected and corrective voice prompts issued
Battery
Item numbers: Standard: M5070A Aviation: 989803139301 (TSO-C142, U.S. only)
Type: 9 Volt DC, 4.2 Ah, lithium manganese dioxide, disposable long-life primary cell
Capacity: When new, a minimum of 200 shocks or 4 hours of operating time at 25° C (77° F)
Install-by date: Battery is labeled with an install-by date of at least five years from date of manufacture
Standby life: Typically, four years when stored and maintained according to directions provided in the Instructions for Use
SMART Pads II
Item number: 989803139261
Active surface area: 80 cm2 (12.4 in2) each
Cable length: 121.9 cm (48 in)
Use-by date: Pads case is labeled with a use-by date of at least two years from date of manufacture
Infant/Child Key: Item number: 989803139311
Training Pads II
Item number: 989803139271
Function: Special pads place the FRx into training mode and disable its energy delivery
capability; features eight real-world training scenarios
Automated and user-activated self-tests
Daily automatic self-tests: Tests internal circuitry, waveform delivery system, pads and battery capacity
Pads integrity test: Specifically tests readiness-for-use of pads (gel moisture)
Battery insertion test: Upon battery insertion, extensive automatic self-tests and user interactive test check device readiness
Status indicators: Blinking green “Ready” light indicates ready for use; audible “chirp” indicates
need for maintenance

Philips HeartStart FRx Defibrillator with Pads
SKU: AED360-861304
Price:
Description
Specification ss
https://www.portableoxygen.co.uk/intermedicaladmin/cms/wysiwyg/directive/___directive/e3ttZWRpYSB1cmw9IjxwPjxzdHJvbmc-RGVmaWJyaWxsYXRvcjwvc3Ryb25nPjwvcD4NCjxwPkhlYXJ0U3RhcnQgRlJ4IEFFRCBzcGVjaWZpY2F0aW9uczo8YnIgLz48YnIgLz5Nb2RlbCBudW1iZXI6IDg2MTMwNDxiciAvPkluY2x1ZGVzIGRlZmlicmlsbGF0b3IsIGJhdHRlcnksIFNNQVJUIFBhZHMgSUkgKDEgc2V0KSwgU2V0dXAgYW5kIE1haW50ZW5hbmNlIEd1aWRlcywgT3duZXImcnNxdW87cyBNYW51YWwsIFF1aWNrIFJlZmVyZW5jZSBHdWlkZSwgZGF0ZSBzdGlja2VyPGJyIC8-PGJyIC8-V2F2ZWZvcm06IEJpcGhhc2ljIHRydW5jYXRlZCBleHBvbmVudGlhbDsgd2F2ZWZvcm0gcGFyYW1ldGVycyBhcmUgYXV0b21hdGljYWxseSBhZGp1c3RlZCBhcyBhPGJyIC8-ZnVuY3Rpb24gb2YgcGF0aWVudCBkZWZpYnJpbGxhdGlvbiBpbXBlZGFuY2U8L3A-DQo8cD48YnIgLz5UaGVyYXB5OiBBZHVsdCBkZWZpYnJpbGxhdGlvbjogbm9taW5hbCBwZWFrIGN1cnJlbnQgMzIgQSAoMTUwIEogbm9taW5hbCBpbnRvIGEgNTAgb2htIGxvYWQpIFBlZGlhdHJpYyBkZWZpYnJpbGxhdGlvbiB3aXRoIG9wdGlvbmFsIEZSeCBJbmZhbnQvQ2hpbGQgS2V5IGluc3RhbGxlZDogbm9taW5hbCBwZWFrIGN1cnJlbnQgMTkgQSAoNTAgSiBub21pbmFsIGludG8gNTAgb2htIGxvYWQpPC9wPg0KPHA-PGJyIC8-UHJvdG9jb2w6IERldmljZSBmb2xsb3dzIHByZWNvbmZpZ3VyZWQgc2V0dGluZ3M7IGRlZmlicmlsbGF0aW9uIGFuZCBDUFIgcHJvdG9jb2wgY2FuIGJlIGN1c3RvbWl6ZWQgdXNpbmcgSGVhcnRTdGFydCBFdmVudCBSZXZpZXcgUHJvIHNvZnR3YXJlPC9wPg0KPHA-PGJyIC8-PHN0cm9uZz5Vc2VyIGludGVyZmFjZTwvc3Ryb25nPjwvcD4NCjxwPjxiciAvPkluc3RydWN0aW9uczogRGV0YWlsZWQgdm9pY2UgcHJvbXB0cyBhbmQgdmlzdWFsIGljb25zIGd1aWRlIHJlc3BvbmRlciB0aHJvdWdoIHVzZSBvZiB0aGUgZGVmaWJyaWxsYXRvcjwvcD4NCjxwPjxiciAvPkNQUiBndWlkYW5jZTogVmVyYmFsIGluc3RydWN0aW9ucyBmb3IgYWR1bHQgYW5kIGluZmFudC9jaGlsZCBDUFIgcHJvdmlkZSBpbnN0cnVjdGlvbnMgYW5kIGF1ZGlvIGN1ZXMgZm9yIHRoZSBhcHByb3ByaWF0ZSBudW1iZXIsIHJhdGUgYW5kIGRlcHRoIG9mIGNoZXN0IGNvbXByZXNzaW9ucywgYXMgd2VsbCBhcyBmb3IgZWFjaCBicmVhdGg8L3A-DQo8cD48YnIgLz5Db250cm9sczogR3JlZW4gT24vT2ZmIGJ1dHRvbiwgYmx1ZS1saXQgaS1idXR0b24sIG9yYW5nZSBTaG9jayBidXR0b24sIG9wdGlvbmFsIEluZmFudC9DaGlsZCBLZXkgSW5kaWNhdG9ycyBSZWFkeSBsaWdodCwgYmx1ZS1saXQgaS1idXR0b24sIGNhdXRpb24gbGlnaHQsIGlsbHVtaW5hdGVkIHBhZHMsIGljb25zOyBTaG9jayBidXR0b24gbGlnaHRzIHVwIHdoZW4gc2hvY2sgaXMgYWR2aXNlZDwvcD4NCjxwPjxiciAvPjxzdHJvbmc-UGh5c2ljYWw8L3N0cm9uZz48L3A-DQo8cD48YnIgLz5TaXplOiAxOCB4IDYgeCAyMiBjbSAoNy4xIHggMi40IHggOC44IGluKSBIIHggRCB4IFc8L3A-DQo8cD48YnIgLz5XZWlnaHQ6IEFwcHJveGltYXRlbHkgMS42IGtnICgzLjUgbGIpIHdpdGggYmF0dGVyeSBhbmQgcGFkcyBpbnN0YWxsZWQ8L3A-DQo8cD48YnIgLz48c3Ryb25nPkVudmlyb25tZW50YWwvcGh5c2ljYWwgcmVxdWlyZW1lbnRzPC9zdHJvbmc-PC9wPg0KPHA-PGJyIC8-U2VhbGluZzogV2F0ZXJqZXQtcHJvb2YgSVBYNSBwZXIgSUVDNjA1Mjk8YnIgLz5EdXN0LXByb3RlY3RlZCBJUDVYIHBlciBJRUM2MDUyOTwvcD4NCjxwPjxiciAvPlRlbXBlcmF0dXJlOiBPcGVyYXRpbmcvU3RhbmRieTogMCZkZWc7ICZuZGFzaDsgNTAmZGVnOyBDICgzMiZkZWc7ICZuZGFzaDsgMTIyJmRlZzsgRik8YnIgLz5UcmFuc2llbnQgb3BlcmF0aW5nIChmb3IgMjAgbWludXRlcyBvciBsZXNzLCBhZnRlciByYXBpZCB0cmFuc2l0aW9uIGZyb20gMjAmZGVnOyBDIFs2OCZkZWc7IEZdKTogLTIwIHRvIDUwJmRlZzsgQyAoLTQmZGVnOyB0byAxMjImZGVnOyBGKTsgdW5kZXIgbm9uLWNvbmRlbnNpbmcgaHVtaWRpdHkgY29uZGl0aW9uczwvcD4NCjxwPjxiciAvPkFsdGl0dWRlOiAwIHRvIDQsNTcyIG0gKDAgdG8gMTUsMDAwIGZ0KTwvcD4NCjxwPjxiciAvPkFpcmNyYWZ0OiBNZWV0cyBSVENBL0RPLTE2MCBTZWN0aW9uIDIxIChDYXRlZ29yeSBNICZuZGFzaDsgUmFkaWF0ZWQgRW1pc3Npb25zKSBhbmQgU2VjdGlvbiAyMDxiciAvPihDYXRlZ29yeSBNICZuZGFzaDsgQ29uZHVjdGVkIEltbXVuaXR5LCBhbmQgQ2F0ZWdvcnkgRCAmbmRhc2g7IFJhZGlhdGVkIEltbXVuaXR5KTwvcD4NCjxwPjxiciAvPkNydXNoOiA1MDAga2cgKDExMDAgbGIpPC9wPg0KPHA-PGJyIC8-RHJvcDogV2l0aHN0YW5kcyAxLjIyIG0gKDQgZnQpIGRyb3Agb24gYW55IGVkZ2UsIGNvcm5lciBvciBmYWNlIG9mIHRoZSBkZXZpY2Ugb250byBtYXNvbnJ5IHN1cmZhY2U8L3A-DQo8cD48YnIgLz5WaWJyYXRpb246IE9wZXJhdGluZzogbWVldHMgTUlMU1REIDgxMEcgRmlnLiA1MTQ2RS0xLCByYW5kb208YnIgLz5TdGFuZGJ5OiBtZWV0cyBNSUxTVEQgODEwRyBGaWcuIDUxNDZFLTIsIHN3ZXB0IHNpbmUgKGhlbGljb3B0ZXIpPC9wPg0KPHA-PGJyIC8-RU1JOiAocmFkaWF0ZWQvIGltbXVuaXR5KSBNZWV0cyBDSVNQUiAxMSBHcm91cCAxIENsYXNzIEIgYW5kIElFQyA2MTAwMC00LTM8L3A-DQo8cD48YnIgLz48c3Ryb25nPkRhdGEgcmVjb3JkaW5nIGFuZCB0cmFuc21pc3Npb248L3N0cm9uZz48L3A-DQo8cD48YnIgLz5JbmZyYXJlZDogV2lyZWxlc3MgdHJhbnNtaXNzaW9uIG9mIGV2ZW50IGRhdGEgdG8gYSBQQyB1c2luZyB0aGUgSXJEQSBwcm90b2NvbDwvcD4NCjxwPjxiciAvPkhlYXJ0U3RhcnQgRXZlbnQgUmV2aWV3IFBybyBzb2Z0d2FyZTogRGF0YSBtYW5hZ2VtZW50IHNvZnR3YXJlIChvcHRpb25hbCkgZm9yIGRvd25sb2FkIGFuZCByZXZpZXcgb2YgZGF0YSByZXRyaWV2ZWQgdGhyb3VnaCBkZWZpYnJpbGxhdG9yJnJzcXVvO3MgaW5mcmFyZWQgZGF0YSBwb3J0PC9wPg0KPHA-PGJyIC8-RGF0YSBzdG9yZWQ6IEZpcnN0IDE1IG1pbnV0ZXMgb2YgRUNHIGFuZCB0aGUgZW50aXJlIGluY2lkZW50JnJzcXVvO3MgZXZlbnRzIGFuZCBhbmFseXNpcyBkZWNpc2lvbnM8YnIgLz48YnIgLz48c3Ryb25nPlBhdGllbnQgYW5hbHlzaXMgc3lzdGVtPC9zdHJvbmc-PC9wPg0KPHA-PGJyIC8-UGF0aWVudCBhbmFseXNpczogRXZhbHVhdGVzIHBhdGllbnQgRUNHIHRvIGRldGVybWluZSBpZiBhIHJoeXRobSBpcyBzaG9ja2FibGUuIFJoeXRobXMgY29uc2lkZXJlZCBzaG9ja2FibGUgYXJlIHZlbnRyaWN1bGFyIGZpYnJpbGxhdGlvbiAoVkYpIGFuZCBjZXJ0YWluIHZlbnRyaWN1bGFyIHRhY2h5Y2FyZGlhcyAoVlQpIGFzc29jaWF0ZWQgd2l0aCBsYWNrIG9mIGNpcmN1bGF0aW9uLiBGb3Igc2FmZXR5IHJlYXNvbnMsIHNvbWUgVlQgcmh5dGhtcyBhc3NvY2lhdGVkIHdpdGggY2lyY3VsYXRpb24gd2lsbCBub3QgYmUgaW50ZXJwcmV0ZWQgYXMgc2hvY2thYmxlLCBhbmQgc29tZSB2ZXJ5IGxvdy1hbXBsaXR1ZGUgb3IgbG93LWZyZXF1ZW5jeSByaHl0aG1zIHdpbGwgbm90IGJlIGludGVycHJldGVkIGFzIHNob2NrYWJsZSBWRi48L3A-DQo8cD48YnIgLz5TZW5zaXRpdml0eS9zcGVjaWZpY2l0eTogTWVldHMgQUFNSSBERjgwIGd1aWRlbGluZXMgYW5kIEFIQSByZWNvbW1lbmRhdGlvbnMgZm9yIGFkdWx0IGRlZmlicmlsbGF0aW9uPC9wPg0KPHA-PGJyIC8-U2hvY2sgYWR2aXNlZDogQWJsZSB0byBkZWxpdmVyIGEgc2hvY2sgYXMgc29vbiBhcyB0aGUgZGV2aWNlIGluZGljYXRlcyBhIHNob2NrIGlzIGFkdmlzZWQ8L3A-DQo8cD48YnIgLz5RdWljayBTaG9jazogQWJsZSB0byBkZWxpdmVyIGEgc2hvY2sgYWZ0ZXIgdGhlIGxhc3QgY2hlc3QgY29tcHJlc3Npb24gb2YgYSBDUFIgaW50ZXJ2YWwsIHR5cGljYWxseSBpbiA4IHNlY29uZHM8L3A-DQo8cD48YnIgLz5TaG9jay10by1zaG9jayBjeWNsZSB0aW1lOiBUeXBpY2FsbHkgbGVzcyB0aGFuIDIwIHNlY29uZHMgYmV0d2VlbiBzaG9ja3MgaW4gYSBzZXJpZXM8L3A-DQo8cD48YnIgLz5BcnRpZmFjdCBkZXRlY3Rpb246IEFsbG93cyBFQ0cgYW5hbHlzaXMgZXZlbiBpbiB0aGUgcHJlc2VuY2Ugb2YgbW9zdCBwYWNlbWFrZXIgYXJ0aWZhY3QgYW5kIGVsZWN0cmljYWwgbm9pc2Ugc291cmNlczsgb3RoZXIgYXJ0aWZhY3RzIGFyZSBkZXRlY3RlZCBhbmQgY29ycmVjdGl2ZSB2b2ljZSBwcm9tcHRzIGlzc3VlZDwvcD4NCjxwPjxiciAvPjxzdHJvbmc-QmF0dGVyeTwvc3Ryb25nPjwvcD4NCjxwPjxiciAvPkl0ZW0gbnVtYmVyczogU3RhbmRhcmQ6IE01MDcwQSBBdmlhdGlvbjogOTg5ODAzMTM5MzAxIChUU08tQzE0MiwgVS5TLiBvbmx5KTwvcD4NCjxwPjxiciAvPlR5cGU6IDkgVm9sdCBEQywgNC4yIEFoLCBsaXRoaXVtIG1hbmdhbmVzZSBkaW94aWRlLCBkaXNwb3NhYmxlIGxvbmctbGlmZSBwcmltYXJ5IGNlbGw8L3A-DQo8cD48YnIgLz5DYXBhY2l0eTogV2hlbiBuZXcsIGEgbWluaW11bSBvZiAyMDAgc2hvY2tzIG9yIDQgaG91cnMgb2Ygb3BlcmF0aW5nIHRpbWUgYXQgMjUmZGVnOyBDICg3NyZkZWc7IEYpPC9wPg0KPHA-PGJyIC8-SW5zdGFsbC1ieSBkYXRlOiBCYXR0ZXJ5IGlzIGxhYmVsZWQgd2l0aCBhbiBpbnN0YWxsLWJ5IGRhdGUgb2YgYXQgbGVhc3QgZml2ZSB5ZWFycyBmcm9tIGRhdGUgb2YgbWFudWZhY3R1cmU8L3A-DQo8cD48YnIgLz5TdGFuZGJ5IGxpZmU6IFR5cGljYWxseSwgZm91ciB5ZWFycyB3aGVuIHN0b3JlZCBhbmQgbWFpbnRhaW5lZCBhY2NvcmRpbmcgdG8gZGlyZWN0aW9ucyBwcm92aWRlZCBpbiB0aGUgSW5zdHJ1Y3Rpb25zIGZvciBVc2U8L3A-DQo8cD48YnIgLz48c3Ryb25nPlNNQVJUIFBhZHMgSUk8L3N0cm9uZz48L3A-DQo8cD48YnIgLz5JdGVtIG51bWJlcjogOTg5ODAzMTM5MjYxPC9wPg0KPHA-PGJyIC8-QWN0aXZlIHN1cmZhY2UgYXJlYTogODAgY20yICgxMi40IGluMikgZWFjaDwvcD4NCjxwPjxiciAvPkNhYmxlIGxlbmd0aDogMTIxLjkgY20gKDQ4IGluKTwvcD4NCjxwPjxiciAvPlVzZS1ieSBkYXRlOiBQYWRzIGNhc2UgaXMgbGFiZWxlZCB3aXRoIGEgdXNlLWJ5IGRhdGUgb2YgYXQgbGVhc3QgdHdvIHllYXJzIGZyb20gZGF0ZSBvZiBtYW51ZmFjdHVyZTwvcD4NCjxwPjxiciAvPkluZmFudC9DaGlsZCBLZXk6IEl0ZW0gbnVtYmVyOiA5ODk4MDMxMzkzMTE8L3A-DQo8cD48YnIgLz48c3Ryb25nPlRyYWluaW5nIFBhZHMgSUk8L3N0cm9uZz48L3A-DQo8cD48YnIgLz5JdGVtIG51bWJlcjogOTg5ODAzMTM5MjcxPC9wPg0KPHA-PGJyIC8-RnVuY3Rpb246IFNwZWNpYWwgcGFkcyBwbGFjZSB0aGUgRlJ4IGludG8gdHJhaW5pbmcgbW9kZSBhbmQgZGlzYWJsZSBpdHMgZW5lcmd5IGRlbGl2ZXJ5PGJyIC8-Y2FwYWJpbGl0eTsgZmVhdHVyZXMgZWlnaHQgcmVhbC13b3JsZCB0cmFpbmluZyBzY2VuYXJpb3M8L3A-DQo8cD48YnIgLz48c3Ryb25nPkF1dG9tYXRlZCBhbmQgdXNlci1hY3RpdmF0ZWQgc2VsZi10ZXN0czwvc3Ryb25nPjwvcD4NCjxwPjxiciAvPkRhaWx5IGF1dG9tYXRpYyBzZWxmLXRlc3RzOiBUZXN0cyBpbnRlcm5hbCBjaXJjdWl0cnksIHdhdmVmb3JtIGRlbGl2ZXJ5IHN5c3RlbSwgcGFkcyBhbmQgYmF0dGVyeSBjYXBhY2l0eTwvcD4NCjxwPjxiciAvPlBhZHMgaW50ZWdyaXR5IHRlc3Q6IFNwZWNpZmljYWxseSB0ZXN0cyByZWFkaW5lc3MtZm9yLXVzZSBvZiBwYWRzIChnZWwgbW9pc3R1cmUpPC9wPg0KPHA-PGJyIC8-QmF0dGVyeSBpbnNlcnRpb24gdGVzdDogVXBvbiBiYXR0ZXJ5IGluc2VydGlvbiwgZXh0ZW5zaXZlIGF1dG9tYXRpYyBzZWxmLXRlc3RzIGFuZCB1c2VyIGludGVyYWN0aXZlIHRlc3QgY2hlY2sgZGV2aWNlIHJlYWRpbmVzczwvcD4NCjxwPjxiciAvPlN0YXR1cyBpbmRpY2F0b3JzOiBCbGlua2luZyBncmVlbiAmbGRxdW87UmVhZHkmcmRxdW87IGxpZ2h0IGluZGljYXRlcyByZWFkeSBmb3IgdXNlOyBhdWRpYmxlICZsZHF1bztjaGlycCZyZHF1bzsgaW5kaWNhdGVzPGJyIC8-bmVlZCBmb3IgbWFpbnRlbmFuY2U8L3A-In19/key/ea7a7b81512c44d08e6a4f2852de80499c118515330b1db8e449e6361c0478b5/
Images